Be aware that the steps outlined in this article may not be suitable for all food businesses, especially those that are large or operate in an unconventional manner. Also, there are a number of preparatory stages that we advise all food businesses to work through before completing a HACCP plan - to find out more about these stages, consider purchasing our HACCP Principles training course.
What are the HACCP principles?
There are seven HACCP principles that must be worked through to create a HACCP plan. These principles are:
- Conduct a hazard analysis
- Identify and list potential hazards
- Analyse the hazards
- Specify control measures
- Determine CCPs (Critical Control Points)
- Establish critical limits
- Establish a monitoring system
- Establish a corrective action plan
- Validation, verification and review
- Documentation and record-keeping
We will look at each of these principles in turn throughout this article.
HACCP Principle 1a - Identify and list potential hazards
The first HACCP principle is split into three by the Food Standards Agency to make it easier to complete correctly. In principle 1a, all hazards that could occur during the food production process should be identified.
All of the hazards that could reasonably occur while producing food should be considered including:
- Cross contamination caused by sharing equipment with contaminated food.
- Contamination with physical hazards, such as jewellery, or chemical hazards, such as cleaning solutions.
Once a list of hazards has been created, it should be established how and where each hazard could cause harm. Some important things to consider include:
- Whether the hazard is present in any raw materials used.
- Whether there are points in the food production process where hazards may be introduced, survive, multiply or increase in frequency.
HACCP Principle 1b - Analyse the hazards
Once a list of potential hazards has been created, they each need to be analysed to determine their significance and identify those requiring control measures. To help with this, ensure that each hazard identified in principle 1a has a brief description to accompany it.
Once this has been completed, a ‘severity’ and ‘likelihood’ score must be assigned to each hazard. Each of these scores should be between 1 and 4, with 1 indicating a low severity or likelihood, and 4 indicating a high severity or likelihood.
The table below provides some information to assist with assigning likelihood and severity scores for each hazard. Be aware that, while the use of scores between 1 and 4 is recommended, some businesses may use different scales.
| Likelihood | Severity |
1 | It is improbable that a hazardous event will occur. | There is little risk of significant harm to the consumer. These hazards often concern product quality more than safety. |
2 | The chance of an event occurring is unlikely, but still possible. | The hazard could cause harm to a consumer such as a short-term illness or small cuts. |
3 | It is reasonably likely that a hazardous event will happen. | The hazard could cause serious harm, such as a long-term illness, but will not result in death. |
4 | A hazardous event is likely to happen. | The hazard could cause significant harm, such as food poisoning and internal bleeding, or death. |
Once likelihood and severity scores have been assigned, a significance rating (between 1 and 16) can be calculated for each hazard by multiplying them together:
Significance = Likelihood x Severity
At this point, the hazards that are significant and will be controlled by the HACCP plan must be decided upon. This is done by selecting a significance score to use as a cut-off, with any hazards that score above this value being controlled by the HACCP plan.
Any hazards that score below this cut-off value must already be controlled by existing preventative measures, such as an existing cleaning schedule or uniform requirement.
HACCP Principle 1c - Specify control measures
At this point, all of the potential hazards present in the food production process should have been identified and sorted to determine which of them are significant. Principle 1c requires suitable control measures (and ways to monitor them) to be decided upon that eliminate these significant hazards or reduce them to an acceptable level. Be aware that:
- Multiple control methods may be required to manage one hazard.
- One control measure may manage numerous hazards.
- A control measure used to control a specific hazard may not be implemented at the same step at which the hazard arises.
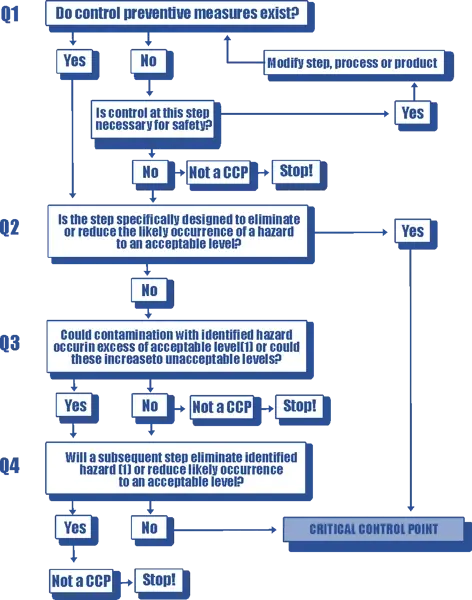
HACCP Principle 2 - Determine CCPs (Critical Control Points)
As previously mentioned, a critical control point is a point in the food production process where a control measure must be applied to eliminate or reduce a food safety hazard.
Identifying critical control points is usually done using the Codex decision tree, which is shown below:
![HACCP Plan Infographic HACCP Plan Infographic]()
HACCP Principle 3 - Establish critical limits
In this principle, critical limits for each critical control point (CCP) must be established. These are values that can be audited against to ensure adequate control is maintained. Appropriate critical limits should be:
- Observable - Achievement of, and changes to, the critical limit can be detected.
- Measurable - Achievement of, and changes to, the critical limit can be measured and quantified.
- Monitorable in real-time/at the point of processing.
The nature of a critical limit will vary depending on the CCP to which it applies. Some examples include:
- pH
- Temperature
- Time
- Weight
- Absence of metal
Where possible, this limit should be determined by relevant legislation or guidance and the method by which it was determined should be recorded. It may also be appropriate to decide on a target value, which is more strict than the critical value and allows for tolerances to be built into the system.
Often, a combination of critical limits will be used to monitor a single control point. For example, when cooking chicken, both the maximum temperature it reaches and the length of time it remains at this temperature should be checked.
HACCP Principle 4 - Establish a monitoring system
After deciding on critical values, a system needs to be put in place to monitor these values and ensure that all control measures function as intended. The method and frequency of monitoring will be determined by the nature of the product or process being monitored.
For example, measuring the temperature of a liquid ingredient would lend itself well to constant in-line monitoring, whereas determining the salt content of a product would likely require less frequent off-line analysis.
The method and frequency of monitoring will also be determined in part by the technology available and how appropriate it is to implement it.
The monitoring system that is established should be documented as part of the HACCP plan, including what is being monitored, who will monitor it, how it is monitored and how often, and the records that must be kept.
HACCP Principle 5 - Establish a corrective action plan
This principle requires businesses to establish a corrective action plan, which is a set of pre-planned actions to be taken in the event that control is lost at a CCP. Corrective actions can be split into two types:
- Corrective actions designed to prevent loss of control, that are used when a monitoring system identifies a failure to reach target values but the critical value itself has not yet been breached.
- Corrective actions designed to regain control, that are used when the critical value has been breached. These actions should restore control of the system, identify and control any affected product(s) and investigate the reason for the loss of control.
When deciding on corrective actions, the following should be considered:
- What immediate actions need to be taken?
- What is going to happen to any products processed since the last known point at which the CCP was under control?
- What changes need to be made to stop the event from recurring?
- Who is responsible for carrying out the corrective action, and are they trained and competent to do so?
The HACCP plan should document the corrective actions that have been decided upon. If these actions are taken, the incident that triggered the corrective action must be recorded along with details of who undertook the work and when.
HACCP Principle 6 - Validation, verification and review
This principle requires a business to check that the HACCP plan they have created will produce safe food and effectively manage the hazards present. There are three main stages to this:
- Validation: will the HACCP plan work?
- Verification: is the HACCP plan working?
- Review: is the HACCP plan up to date?
Validation
This is the process by which the decisions made in creating the HACCP plan are confirmed. The FSA recommend that the following information is recorded for each principle:
- Principle 1: The plan should reference an authority (such as a journal or other guidance) for each identified hazard, justify the evaluation process used and provide an explanation for why any identified hazards were not included in the final list.
- Principle 2: The plan should specify which method was used to determine the critical control points.
- Principle 3: The plan should explain how each critical value was chosen, either by referencing relevant guidance or providing suitable validation exercises, and demonstrate that the process is capable of operating at the proposed critical limit.
- Principle 5: If a corrective action includes a plan to reuse a non-conforming product, the plan should explain how the product will be made safe to eat.
Verification
At this point, it must be checked that the HACCP plan that has been created is working in practice and that its critical values are sufficient to protect the food produced. The steps involved in doing so will vary depending on the food production process, but may include:
- Taking measurements at various points throughout the production process.
- Taking samples of the final product to ensure that it meets microbiological/chemical standards.
- Auditing documentation to ensure that records are being kept correctly.
- Staff assessments to ensure that they are trained and competent.
- External audits of suppliers to ensure that they meet the standards required.
Review
For a HACCP plan to be effective, it must be kept up to date. The plan should be reviewed at least annually, and when an influential change makes a review necessary such as:
- Changes in the ingredients and raw materials used.
- The introduction of a new product or process.
- A change in supplier.
- Changes to the processing system or environment.
- Failures in the system.
- Changes in legislation.
All reviews, validation studies and verification studies must be documented to prove due-diligence if required.
HACCP Principle 7 - Documentation and record-keeping
Maintaining good records is essential in a food business because they provide proof that the HACCP plan is working correctly and that sufficient measures are being taken to ensure that all food produced is safe.
This means that a business’s documentation and record-keeping practices must be appropriate to its business's size and be adequate to prove that the HACCP plan is implemented and maintained correctly.
Some essential questions to consider regarding documentation include:
- What records need to be kept?
- How should they be stored (electronically or physically)?
- Where should they be stored?
- How long should the records be kept?
- Who is responsible for keeping and maintaining the records?
- Who needs to be able to access the documents?
Some example documentation and records are shown below:
Documentation | Records |
The HACCP plan | CCP monitoring activities |
A hazard analysis | Corrective actions taken |
CCP determination | Regular inspection reports |
Standard operating procedures | Training records |
At Commodious, we offer several training courses designed to help food businesses to protect their customers from experiencing harm caused by the food they sell. To find out more about these courses, use the links below: